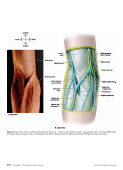
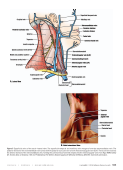
The Art and Science of Infusion Nursing The Art and Science of Infusion Nursing S8 Copyright © 2016 Infusion Nurses Society Journal of Infusion Nursing M E T H O D O L O G Y F O R D E V E L O P I N G T H E S T A N D A R D S O F P R A C T I C E Role of the Standards of Practice Committee The Standards of Practice Committee brought together a group of professional nurses with a wealth of clinical knowledge and expertise in all the domains of infusion therapy. They initially met to review and agree on the evidence rating scale and to discuss methods and sources of searching for evidence. They also agreed on how to evaluate types of evidence. Throughout the Standards review and revision process, the committee met regularly by phone, reviewed each standard in detail, and came to consensus on the final strength of the body of evidence rating for the final draft of the Infusion Therapy Standards of Practice . This draft then was sent to over 90 interdisciplinary reviewers who are experts in the field, comprising all aspects of infusion therapy. Sixty reviewers provided in excess of 790 comments, suggestions, refer- ences, and questions. The committee addressed each com- ment and made revisions to the standards, seeking addi- tional evidence as needed. Each standard had a final review by the committee for agreement on the content, evidence, recommendation, and rating. The standards are written for clinicians of multiple disciplines with various educational backgrounds, train- ing, certification, and licensing, including licensed inde- pendent practitioners, because infusion therapy may be provided by any one of these individuals. The premise is that patients deserve infusion therapy based on the best available evidence, irrespective of the discipline of the clinician who provides that therapy while operating within her or his scope of practice. Searching for Best Evidence A literature search was conducted for each of the stand- ards of practice using key words and subject headings related to the standard. Searches were limited to English-language, peer-reviewed journals published between 2009 and July 2015. Databases included, but were not limited to, Cochrane Library, Cumulative Index to Nursing and Allied Health Literature (CINAHL), MEDLINE, PubMed, and Web of Science. The references of retrieved articles were reviewed for relevant literature. Additional sources of evidence included, but were not limited to, the Web sites of professional organiza- tions, manufacturers, pharmaceutical organizations, and the United States Pharmacopeia (USP). US sites included the US Department of Health and Human Services for national centers, such as the Agency for Healthcare Research and Quality (AHRQ), the Centers for Disease Control and Prevention (CDC), and the US Food and Drug Administration (FDA) and the US Department of Labor (eg, Occupational Safety and Health Administration [OSHA]). Classic papers were included as needed. On occasion, textbooks served as sources of evidence when clinical research and scholarship are widely accepted, such as for anatomy and physiology. Because standards of prac- tice are written for all health care settings and all populations, evidence was included for each of these areas as available. Evaluating Evidence Each item of evidence is evaluated from many perspec- tives, and the highest, most robust evidence relating to the standards of practice is used. Research evidence is preferred over nonresearch evidence. For research evi- dence, the study design is the initial means for ranking. Other aspects of evaluation of quality include sufficient sample size based on a power analysis, appropriate sta- tistical analysis, examination of the negative cases, and consideration of threats to internal and external validity. Research on research, such as meta-analyses and systematic reviews, is the highest level of evidence. Only specific study designs are acceptable for a meta-analysis, and with its statistical analysis, this is the most robust type of evidence. Single studies with strong research designs, such as randomized controlled trials (RCTs), form the basis for research on research or a strong body of evidence when there are several RCTs with similar findings. Other research designs are needed as well for a developing area of science and often before an RCT can be conducted. A necessary and foundational study for learning about a question or a population is the descriptive research project, but because of its lack of research controls, it is ranked at a low level of evidence for clinical practice. JIN-D-15-00057.indd S8 JIN-D-15-00057.indd S8 05/01/16 11:30 PM 05/01/16 11:30 PM